What Best Describes the Action of Immune Checkpoint Inhibitors
This prevents the off signal from being sent allowing the T cells to kill cancer cells. Immune checkpoint inhibitors are a new class of anticancer therapies that amplify T-cell-mediated immune responses against cancer cells.
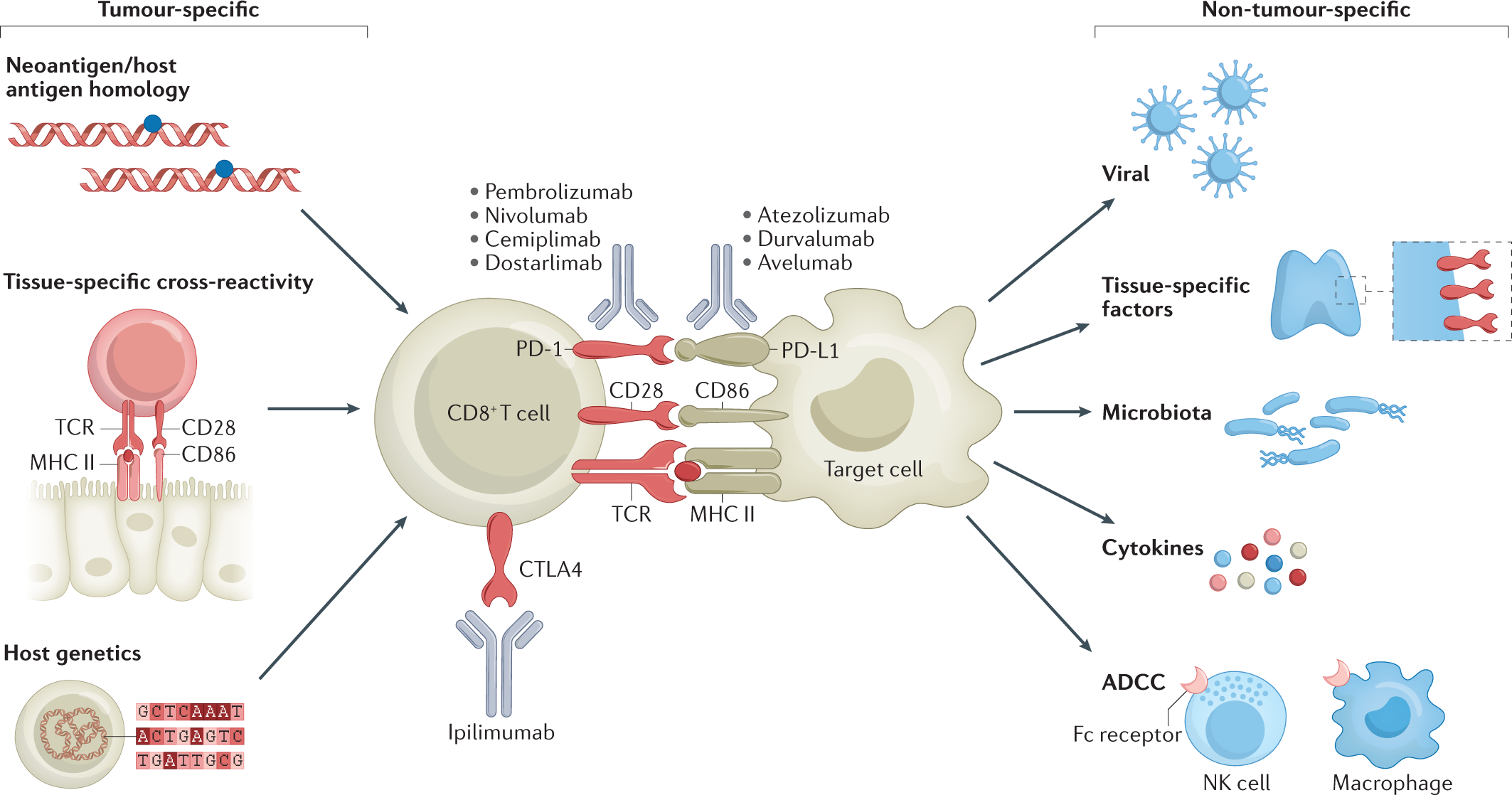
Immune Checkpoint Inhibitors Long Term Implications Of Toxicity Nature Reviews Clinical Oncology
Cancer is one of the most deadly diseases in the modern world.
. These individuals may experience side effects that require urgent treatment and this toolkit will help providers determine the best course of action. However data from a new study indicate that only about 13 of patients will respond to these treatments. Normally when a tumor antigen is presented the immune system activates to target and destroy the tumor.
See The Future Of Multimodal Regimens Built With PD-1 Inhibition Therapy. Hui details the cell biology underlying the action of immune checkpoint inhibitors. These new immunotherapies known as checkpoint inhibitors have revolutionized the treatment of metastatic melanoma.
The immune system works to protect a person from illness by fighting foreign invaders like viruses and bacteria. PD-1 is a checkpoint protein on. Immune Checkpoint Inhibitor Therapy Has Revolutionized Cancer Treatment.
Checkpoint inhibitors dont kill cancer cells directly. They work by helping the immune system to better find and attack the cancer cells wherever they are in the body. A Tumor cells escape immune surveillance by promoting checkpoint activation.
Immune checkpoint inhibitors work by targeting proteins that keep immune responses in check enabling T cells to kill cancer cells more effectively. The percentage of patients with cancer eligible to receive immune checkpoint inhibitors ICIs has increased in recent years to more than 40. Checkpoint inhibitors CPIs have dramatically increased survival in patients with certain types of cancer 1.
The corresponding guideline describes in. Of all the immune checkpoint proteins CTLA-4 is the best understood mechanism. This allows T cells to find and destroy cancer cells.
The PD-1 checkpoint inhibitor nivolumab was associated with an overall survival as long as 25 months in patients with renal cell cancer said Eric Jonasch MD Associate Professor Department of Genitourinary Medical. Immunotherapy has altered the prognosis for several cancers in particular malignant melanoma and nonsmall cell lung cancer. PD-1 and PD-L1 inhibitors.
Medicines that target different checkpoint proteins are now used to treat some types of cancer. Two papers published in Science provide the first clinical evidence that fecal microbial transplants FMTs can boost the anti-cancer efficacy of. Among the properties of OVs is their ability to improve the immune system response to tumors and in combination with checkpoint inhibitors they also enhance checkpoint blockade 116118.
Immunotherapy drugs called immune checkpoint inhibitors work by blocking checkpoint proteins from binding with their partner proteins. Published 16 January 2018. Immune checkpoint inhibitors have shown important benefits in phase 3 trials and several agents have.
By lysing tumor cells OV treatment can lead to the release of tumor-associated antigens by the dying cells and efficient cross-presentation of antigens to DCs thus enhancing anti-tumor. To date 6 CPIs have been approved by the US. Immune checkpoint inhibitors attach to these checkpoints.
Fatal toxic effects associated with immune checkpoint inhibitors. Although many of these agents are showing clinical promise for treating some types of. However immune checkpoint inhibitors ICIs.
Ad Discover the Rapid Clinical Development With Immune Checkpoint Inhibitor Regimens. But rheumatoid arthritis and polymyalgia rheumatica can be added to the list of adverse events. One extremely promising means to achieve anti-cancer immunity is to block the immune checkpoint pathways - mechanisms adopted by cancer cells to d.
All of these drugs are given as an infusion into a vein IV. A systematic review and meta-analysis published online September 13 2018. Food and Drug Administration FDA.
Wang DY Salem J Cohen JV et al. In essence all checkpoint inhibitors take the brakes off of the immune system to enable T-cell proliferation and activation to attack the cancer. Epub 2019 Feb 13.
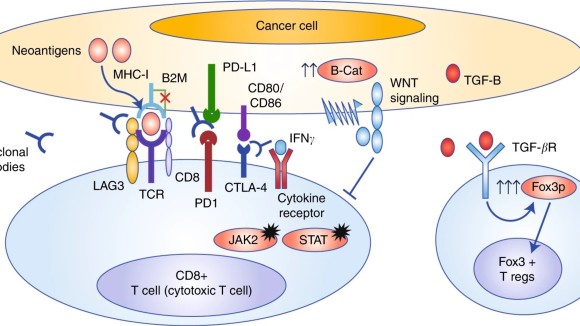
The Immune Checkpoint Inhibitor Toxicity Management Toolkit has been designed to help support individuals taking immune checkpoint inhibitor medications. Mechanism of action of checkpoint inhibitors. Sometimes the immune system is not able to make the distinction between healthy and cancerous cells and fail to recognize a cancer.
Hui details the cell biology underlying the action of immune checkpoint inhibitors. Examples include those that target the programmed cell death protein 1 PD-1 and programmed cell death 1 ligand 1 PD-L1. Immune checkpoint inhibitors J Cell Biol.
Annals of Internal Medicine. However under the CTLA-4 immune checkpoint inhibitors there is only one product currently available ie Yervoy by Bristol-Myers Squibb. Immune checkpoint inhibitor therapy appeared to be a safe effective treatment for patients with advanced-stage cancer and HIV infection according to a systematic review published in JAMA Oncology.
It can also recognize harmful changes happening inside the body like the formation of cancer. An antibody blocking the receptor CTLA4 was the first to show efficacy in treating malignant melanoma 1 followed by antibodies blocking PD1 or its ligand PDL1 2. The last decade has witnessed dramatic advances in cancer treatment through immunotherapy.
They block cancer cells from hitting the off switch. Global immune checkpoint inhibitors marketOverview. In detail they determine an action of releasing the brakes of the immune system where the anti-CTLA4 agents are able to contrast the inactivation of the immune response and to stimulate the induction of an anti-neoplastic immune reaction while the anti-PD1 and anti-PD-L1 drugs act by enhancing the effector activity of T cells in the peripheral tissues most.
Immune checkpoint therapy using a CTLA-4 inhibitor and an antiPD-1 antibody is in ongoing clinical studies in patients with metastatic renal cell cancer. An increase in the number of immune checkpoint inhibitors approved by the FDA along with more indications means that 43 of patients with cancer in the United States are eligible to receive. Ipilimumab is a cytotoxic T lymphocyteassociated protein 4 CTLA-4 inhibitor.
Tumor cells express the immune checkpoint activator programmed cell death ligand 1 PD-L1 and produce antigens blue dots that are captured by antigen-presenting cells APCs.

Immune Checkpoint Inhibitors In Cancer Treatment Notes Inability To Download Scientific Diagram
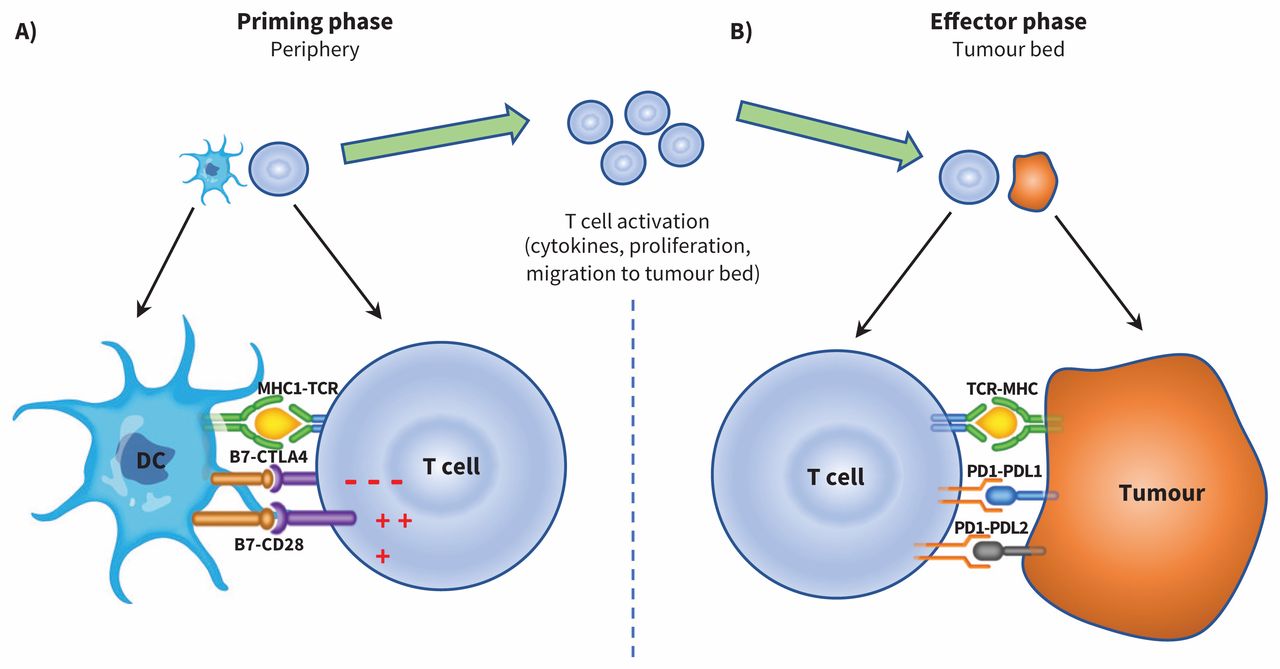
Adverse Events Associated With Immune Checkpoint Inhibitor Treatment For Cancer Cmaj
No comments for "What Best Describes the Action of Immune Checkpoint Inhibitors"
Post a Comment